INTENSE-TBM,CLINICAL SITES,EVENTS
December 2025 – recruitment completed
December 2025: recruitment completed...
INTENSE-TBM,CLINICAL SITES,EVENTS
July 2025 – 700 participants included
July 2025: 700 participants included...
INTENSE-TBM,CLINICAL SITES,EVENTS
July 2025 – coordination team visit in Uganda
July 2025: visit in Uganda to validate causes of death and close the sites...
ANNUAL NUMBER OF TB CASES
ANNUAL NUMBER OF TB DEATHS
ANNUAL NUMBER OF NEW TBM CASES
TBM MORTALITY
In 2017, an estimated 10 million new cases and 1.6 million deaths occurred worldwide due to tuberculosis (TB) (World Health Organization, Global Tuberculosis Report 2018). The most lethal and disabling form of TB is tuberculous meningitis (TBM), with an estimated 100,000 new cases occurring per year, representing around 6% of extra-pulmonary TB cases.
In sub-saharan Africa, TBM mortality reaches 40% in HIV-negative patients and up to 70% in HIV-positive individuals with drug resistant Mycobacterium tuberculosis strains, with death occurring most frequently within the first 2 weeks after diagnosis. Amongst TBM survivors, 50% are disabled due to neurological sequelae.
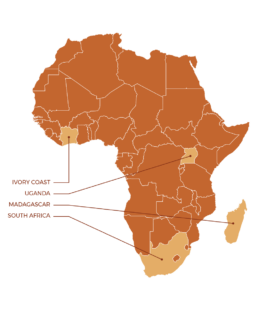
INTENSE-TBM is a five-year international project, funded by EDCTP2 (The European & Developing Countries Clinical Trials Partnership), sponsored by Inserm-ANRS and coordinated by the University of Bordeaux, aiming at improving the overall care of people affected by TBM. It includes a clinical trial in 4 African countries (Ivory Coast, Madagascar, South Africa and Uganda) to evaluate the efficacy of an intensified treatment of tuberculosis and the addition of aspirin, in patients co-infected or not with HIV.
It is expected that this intensified treatment represents a major advance in the management of TBM by reducing the mortality rate by at least 30%, minimizing the neurological sequelae, improving the standards of clinical management of patients co-infected with HIV and ultimately leading to an update of national and international standards for TB care. More broadly, the INTENSE-TBM project will contribute to the improvement of standards of care in Sub-Saharan Africa and its results could impact the clinical management of other severe forms of tuberculosis and in other populations, especially children.
OUTREACH
Tuberculosis is a transmissible infectious disease caused by a microbe called Mycobacterium tuberculosis that is usually transmitted from one person to another through the air when one coughs, sneezes or talks.
Tuberculosis most often affects the lungs, but may also occasionally affect other organs, including the meninges (the system of membranes, which envelops the brain and spinal cord). When the meninges are infected with tuberculosis, we call it « tuberculous meningitis » (TBM). TBM is a serious disease that can cause death or neurological complications (paralysis, speech disorders, memory impairments).
The current « standard treatment » of TBM is the one recommended by the World Health Organization (WHO). It consists of a 9-month treatment with four anti-tuberculous drugs. During the first two months, the treatment includes 4 drugs: isoniazid, rifampicin, ethambutol and pyrazinamide. During the following seven months, it includes only two drugs: isoniazid and rifampicin.
The « standard TBM treatment » is effective to cure TBM in many people, but in some people it is not, partly because the anti-tuberculous drugs do not fully penetrate the meninges. As a result, some people with TBM still die or suffer long-term complications despite treatment. The INTENSE-TBM clinical trial therefore wants to evaluate whether a new treatment (called « intensified TBM treatment ») could increase the number of treatment successes.
The « intensified TBM treatment » has the same total duration (9 months) as the « standard treatment ». It also contains the same drugs as the « standard treatment » during the last 7 months. However, during the first 2 months, it is called « intensified » because it contains the same drugs as the standard treatment plus two additional drugs (linezolid and aspirin) as well as higher doses for one drug (rifampicin).
The aim of this clinical trial is to prove that the intensified TB treatment strategy can reduce of 30% the risk of death TBM-related, and also the neurological damage.
IMPACT
The excepted impact of INTENSE-TBM is to :